All cells in our body need oxygen for aerobic metabolism to create energy efficiently. Anaerobic metabolism occurs in the absence of oxygen. Aerobic metabolism is up to 18 times more efficient than anaerobic metabolism and so lack of oxygen over time (3-6 minutes) causes cell death. Therefore, oxygen is the key to life
At Aurum, we are developing a novel molecule that carries and delivers oxygen independent of haemoglobin. The molecule has the potential to be used in multiple indications where blood supply is compromised and where tissue is short of oxygen. Below is an explanation of how this technology works in acute ischemic stroke (AIS).
Worldwide:
• 1 in 6 people will have a stroke in their lifetime
• 13.7 million suffer a stroke each year
• 5.8 million die as a consequence of stroke each year
What is a Stroke?
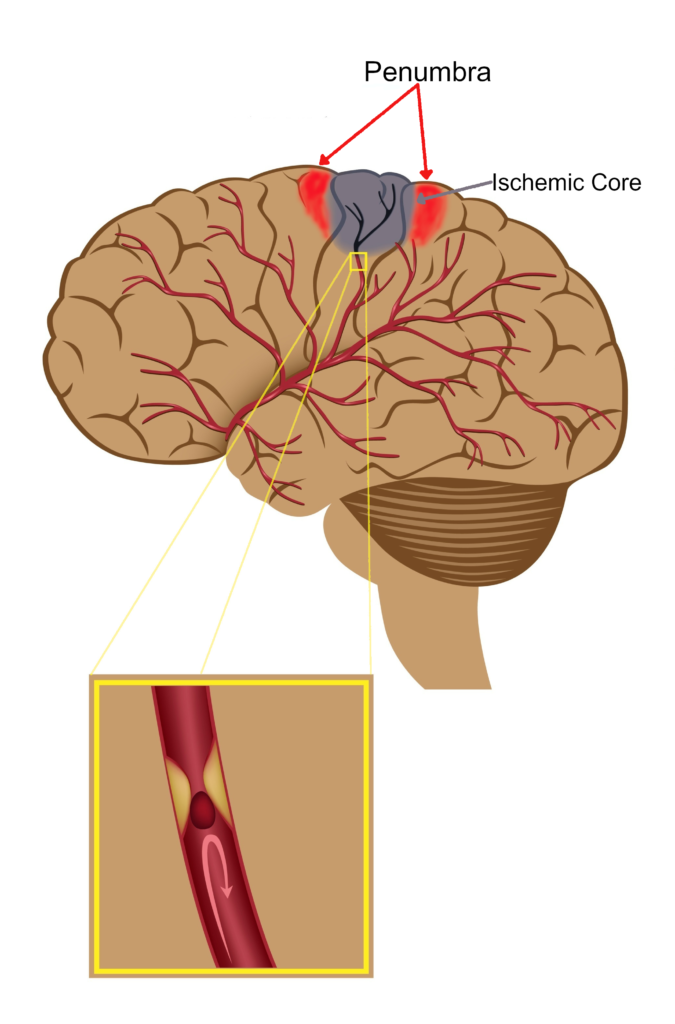
Acute ischaemic stroke ( or Brain Attack) is a serious life-threatening medical condition that happens when the blood supply to part of the brain is blocked by a clot. This results in a lack of oxygen and brain cells are very sensitive to this and some start dying within a few minutes. Estimates are that 1.9 million neurons, 13.4 billion synapses, and 12km of wiring are lost every minute following onset of stroke. When nerve cells die, the area of the body they control can’t work. Therefore, stroke is a medical emergency and urgent treatment is essential.
Current treatments:
Acute therapies in ischaemic stroke are focused on restoring the blood flow. The treatments require rapid interventions for dissolving clots (thrombolysis) and/or removal of clots (mechanical thrombectomy).
Thrombolysis: Some patients with acute ischaemic stroke are eligible for clot-busting therapy also called thrombolysis treatment. Unfortunately, this treatment is time-based and can only be given up to 4.5 hours from the onset of symptoms. However, many patients cannot receive this treatment as the time of stroke onset is not known (wake-up stroke). However, recent studies have shown that conventional Magnetic Resonance Imaging (MRI) in wake-up stroke patients has potential to identify those who can benefit from thrombolysis within 4.5 hours of stroke symptom recognition. Advanced physiological imaging with CT and/or MRI has also shown potential to offer benefit in patients presenting beyond the current 4.5 hour treatment window.
Mechanical Thrombectomy: Mechanical removal of a clot is another treatment for a limited number of patients. Mechanical thrombectomy is indicated for patients with acute ischemic stroke due to a large artery occlusion within 6 hours. With advanced physiological CT and MR Imaging, this time period can be extended up to 24 hours. This is not widely available due to lack of resources and expertise required to support the deliver this therapy.
Problems we are trying to address:
Thrombolysis as a treatment of stroke was licensed more than 25 years ago. Since then, the rate of patients treated with thrombolysis ranges from 1 in 10 to 1 in 100 patients presenting with acute ischaemic stroke. Likewise, only 1 in 10 patients with acute ischemic stroke would be eligible for mechanical thrombectomy within 6 hours, and even less between 6 to 24 hours. Furthermore, only a few stroke centres have sufficient resources and expertise to deliver mechanical thrombectomy.
A minority of acute ischaemic stroke patients are treated with the current techniques worldwide, leaving the vast majority (80% – 99%) untreated. Therefore, there is a need to increase the uptake of the currently available techniques. Furthermore, only between 10-25% of treated patients benefit from current treatments, thus highlighting the urgent need for new therapies to target all patients with acute ischaemic stroke.
Our Solutions :
Developing novel and safe therapy that can be given irrespective of the time window
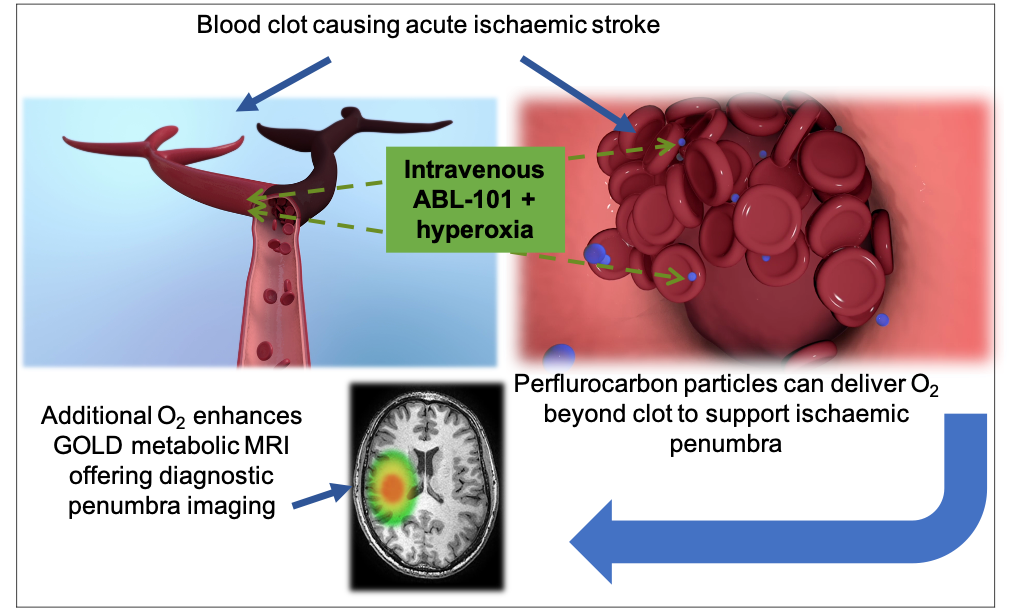
Oxygen is carried by haemoglobin in red blood cells and is vital for aerobic metabolism to maintain life. However, when there is a blood clot blocking an artery as in acute ischaemic stroke within the brain, there is a lack of oxygen delivered to the tissues resulting in the death of brain cells. ABL-101 is a more efficient oxygen carrier than haemoglobin and is also 30-40 times smaller than red blood cells. Therefore, it can bypass the clot and efficiently deliver oxygen to tissues starved of oxygen via alternate small blood vessels (collaterals), thus preventing cell death. ABL-101 has a good safety profile in early clinical studies and could be available to all acute ischaemic stroke patients. It would also work synergistically with the current therapies.
Developing novel metabolic imaging technique for precision medicine
At Aurum, we have also developed novel proprietary MR metabolic imaging techniques called Glasgow Oxygen Level Dependent (GOLD) using ABL-101 to identify salvageable brain tissue (penumbra). As these techniques are based on tissue metabolism they are more accurate in identifying the salvageable tissue than the current advanced imaging techniques based on physiology alone. This will help to identify wake-up stroke patients and also those outside the current therapeutic time windows, who could benefit from current stroke treatments. Therefore, with GOLD imaging technology more patients would benefit from the current therapies.
Developing Novel Imaging Technology For Inflammation
ABL-101 is made up of a large number of fluorine atoms that can be visualised in MRI using a detector that is tuned to fluorine (19F MRI). Once injected, cells that are also part of the inflammatory response (macrophages) take up ABL-101. Therefore, ABL-101 in addition to its use in GOLD imaging and stroke therapy, can be used to identify inflammation in the brain infarct following an ischaemic stroke. 19F MRI could identify active inflammation within the infarct, which is considered a prime target for development of new stroke therapies. It could also identify active inflammation within atherosclerotic plaques that have an increased risk of rupture and could therefore be used to guide treatment in the prevention of further devastating strokes.
Publications :
Santosh C, Brennan D, McCabe C, Macrae IM, Holmes WM, Graham DI, Gallagher L, Condon B, Hadley DM, Muir KW, Gsell W. Potential use of oxygen as a metabolic biosensor in combination with T2*-weighted MRI to define the ischemic penumbra. J Cereb Blood Flow Metab. 2008 Oct;28(10):1742-53. doi: 10.1038/jcbfm.2008.56.
Baskerville TA, Deuchar GA, McCabe C, Robertson CA, Holmes WM, Santosh C, Macrae IM. Influence of 100% and 40% oxygen on penumbral blood flow, oxygen level, and T2*-weighted MRI in a rat stroke model. J Cereb Blood Flow Metab. 2011 Aug;31(8):1799-806. doi: 10.1038/jcbfm.2011.65.
Holmes WM, Lopez-Gonzalez MR, Gallagher L, Deuchar GA, Macrae IM, Santosh C. Novel MRI detection of the ischemic penumbra: direct assessment of metabolic integrity. NMR Biomed. 2012 Feb;25(2):295-304. doi: 10.1002/nbm.1748.
Dani KA, Santosh C, Brennan D, Hadley DM, Muir KW. Crossed cerebellar diaschisis: insights into oxygen challenge MRI. J Cereb Blood Flow Metab. 2012 Dec;32(12):2114-7. doi: 10.1038/jcbfm.2012.142.
Deuchar GA, Brennan D, Griffiths H, Macrae IM, Santosh C. Perfluorocarbons enhance a T2*-based MRI technique for identifying the penumbra in a rat model of acute ischemic stroke. J Cerebral Blood Flow Metab 2013; doi:10.1038/jcbfm.2013.86.
Robertson CA, McCabe C, Lopez-Gonzalez MR, Deuchar GA, Dani K, Holmes WM, Muir KW, Santosh C, Macrae IM. Detection of ischemic penumbra using combined perfusion and T2* oxygen challenge imaging. Int J Stroke. 2015 Jan;10(1):42-50. doi: 10.1111/ijs.12327.
Dani KA, Moreton FC, Santosh C, Lopez R, Brennan D, Schwarzbauer C, Goutcher C, O’Hare K, Macrae IM, Muir KW. Oxygen challenge magnetic resonance imaging in healthy human volunteers. J Cereb Blood Flow Metab. 2017 Jan;37(1):366-376. doi: 10.1177/0271678X15627827.
Deuchar GA, Brennan D, Holmes WM, Shaw M, Macrae IM, Santosh C. Perflurocarbon Enhanced Glasgow Oxygen Level Dependant (GOLD) Magnetic Resonance Metabolic Imaging Identifies the Penumbra Following Acute Ischemic Stroke. Theranostics 2018 Feb 12;8(6):1706-1722 DOI: 10.7150/thno.21685.
Deuchar GA, van Kralingen JC, Work LM, Santosh C, Muir KW, McCabe C, Macrae IM. Preclinical Validation of the Therapeutic Potential of Glasgow Oxygen Level Dependent (GOLD) Technology: a Theranostic for Acute Stroke. Transl Stroke Res. 2019 Oct;10(5):583-595. doi: 10.1007/s12975-018-0679-y.
Darçot E, Colotti R, Brennan D, Deuchar GA, Santosh C, van Heeswijk RB. A characterization of ABL-101 as a potential tracer for clinical fluorine-19 MRI. NMR Biomed. 2020 Jan;33(1):e4212. doi: 10.1002/nbm.4212.
Patents :
Method of Imaging Metabolic Function. US Patent Number 9144392. Granted: 29th September 2015.
Method of Imaging Metabolic Function. EU Patent Number 2053967. Granted 9th May 2018.
Method of Treating Traumatic Brain injury. EU Patent Number 2309849. Granted: 2nd September 2015.
Improved Method of Determining Metabolic Function. US Patent Number 9322895. Granted: 26th April 2016.
Improved Method of Determining Metabolic Function. EU Patent Number 2464286. Granted 8th May 2019.
Improved Method of Assessing Metabolic Function. US Patent Number 10278642. Granted 7th May 2019.